There are various types of vaccines:
- Live-attenuated vaccines
- Inactivated vaccines
- Subunit, recombinant, polysaccharide and conjugate vaccines
- Toxoid vaccines
Immunization is the process by which a person or animal becomes protected against a disease.
TRANSPORTING VACCINES WITH DRY ICE
When transporting vaccines at low temperatures, dry ice can be used. Dry ice is CO2 in its solid phase, carbon dioxide turn into dry ice at –78.5°C (at 1 atm). Dry ice is often used as a coolant due to the fact that it is colder than water ice and that CO2 has no liquid phase in standard atmospheric conditions meaning that when dry ice melts, there is only an increase of CO2 levels but no other residues. As CO2 is an odourless and colourless gas, it is very important for life safety to always monitor the CO2 level when using dry ice. CO2 is capable of causing asphyxiation at high concentrations!
CRITICAL TEMPERATURE RANGE
To ensure the product quality, vaccines are manufactured in sterile environments and temperature-controlled environments. Vaccines are fragile and must be maintained at recommended temperatures, from manufacturing to administration, to ensure that they don’t deteriorate. The temperature range will depend on the vaccines. For example, the Pfizer/BioNTech COVID-19 vaccine must be stored at -70°C, whereas the majority of vaccines can be stored in a fridge between 2…8°C.
REGULATIONS
Various regulations exist to ensure the product quality during the distribution of vaccines:
- EU Good Distribution Practice of medicinal products for human use.
- WHO Annex 9 Model guidance for the storage and transport of time- and temperature–sensitive pharmaceutical products.
- USP 1079 Good Storage and Distribution Practices for Drug Products.
TEMPERATURE MONITORING:
It is important for a facility to have proper storage and monitoring equipment and based upon the GDP regulations, the equipment must be set up correctly, maintained appropriately including a frequent calibration and repaired as needed. This will ensure that the vaccines cold chain is not broken, and the vaccines integrity is not compromised meaning that patients are not receiving compromised vaccines or being vaccine twice and potentially expensive vaccines don’t have to be disposed of.
The Rotronic continuous and configurable environmental monitoring system can monitor all stages of the lifetime of a vaccine: from the initial pharmaceutical quality, via the non-clinical trials, the clinical trials and the scientific evaluation and authorisation, then onto the large-scale production, distribution and storage of vaccines.
THE ROTRONIC MONITORING SYSTEM SOLUTION
The Rotronic Monitoring System (RMS) is a GAMP©5 category 4 software combined with category 1 hardware, helping users monitor their GxP compliant applications, looking into the critical quality attributes and monitoring critical process parameters, helping focus on patient safety, product quality and data integrity and compliant to EudraLex Annex 11 and FDA 21 CFR Part 11.
RMS helps reduce human interaction and reduces risk (every day taking temperature values on paper).
For sterile manufacturing, please see here: CLEAN ROOM & ISOLATORS
For cold chain transport and storage, please see here: Cold Chain
Hardware advantages:
- Monitoring of temperature, relative humidity, lux and door contacts
- Rotronic offer digital data loggers with a configurable measuring intervals between 10s and 60 minutes.
- The data loggers ensure data integrity with a backup battery as well as onboard memory (minimum 7000 values).
- Door monitoring also possible.
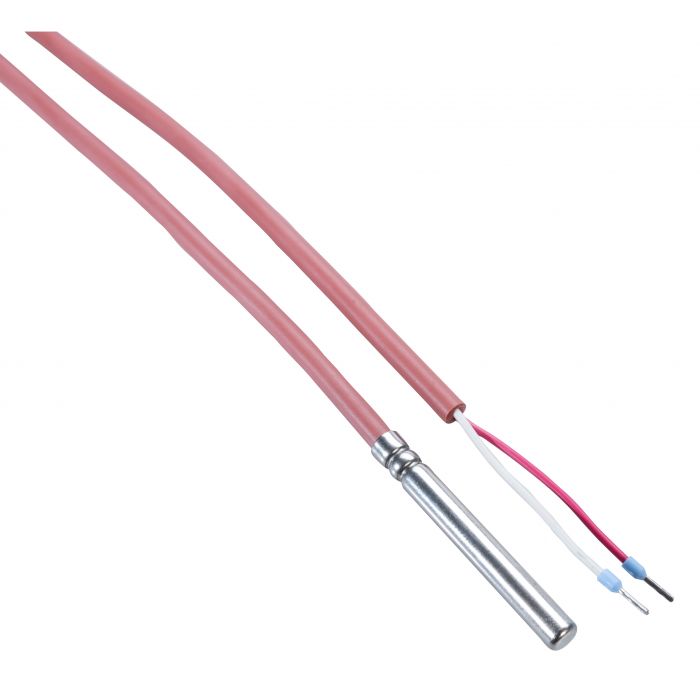
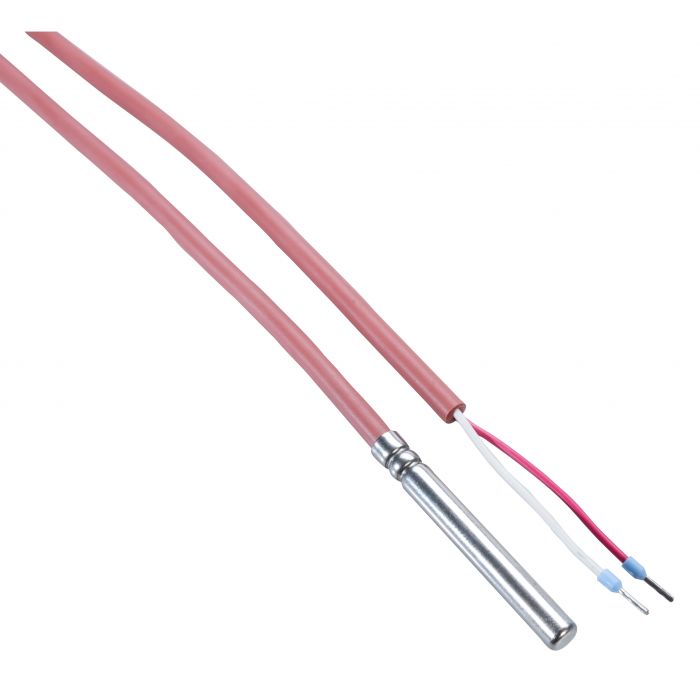
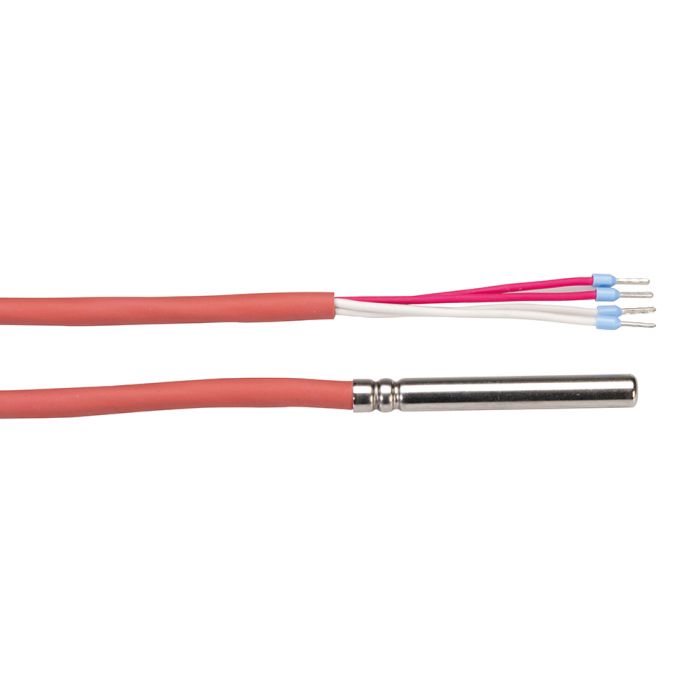
- To ensure that the data logger is representative of vaccine temperature, Rotronic offers a temperature buffer: RMS-TD-0001
- Accuracy better than +/-0.5°C and 5%rh.
Software advantages:
- Visualisation of values and limits
- Alarm for out-of-range temperatures
- Low-battery indicator
- Current, minimum, and maximum temperature display
- Audit trail: Any actions taken if a temperature excursion occurred
- Temperature data should be kept for three years (unless state statutes or rules require a longer period).
- Calibration managment
ROTRONIC SERVICES
Rotronic can offer calibration and qualification of temperature controlled areas and product training.