
The Rotronic Monitoring System can help with 3 critical applications that require environmental monitoring within the healthcare industry:
1. Statutory/Regulations
2. Clinical
3. Personal comfort
In all healthcare environments (hospitals, clinics…) a critical part of the building management system (BMS) is the heating, ventilation and air conditioning (HVAC). The HVAC manages the air supply within a building to ensure specific environmental conditions for specific areas based upon the application carried out within the area:
As air can transport and spread airborne infectious agents it is crucial to ensure that the HVAC is working correctly.
Regulations and recommendations have been put into place to ensure that both patients and staff at not at risk and these are the common areas subjected to various regulations.
•Operating departments
•Laboratories (diagnostic samples)
•Wards
•Pharmacies
•Medical refrigerators and freezers (for vaccines for example)
•Areas containing identified biological or chemical hazards
•Biological safety cabinets (BSC’s)
•Blood, cell and tissue banks
•Areas containing oxygen-displacing gases
•Areas containing medical devices (to avoid electro static discharge (ESD))
•Enclosed work spaces
•Workshops...
The regulations will vary around the world based upon the regulatory authorities, here are only a few of them:
•DIN 1946-4:2018-09 Ventilation and air conditioning - Part 4: Ventilation in buildings and rooms of health care
•ASHRAE Standard 170 Ventilation of Health Care Facilities
•ISO 14644 Cleanrooms and associated controlled environments (for both operation rooms and pharmacy sterile production)...
Within the healthcare industry drugs can be compounded, stored and distributed, requiring for the entity to follow strict specific GxP quality guidelines.
Good Laboratory Practice (GLP): Defines a set of rules and criteria for a quality system concerned with the organisational process and the conditions under which non-clinical health and environmental safety studies are planned, performed, monitored, recorded, reported and archived.
Good Distribution Practice (GDP): Describes the minimum standards that must be met to ensure that the quality and integrity of medicines is maintained throughout the supply chain. GDP applies to sourcing, storage and transportation of active pharmaceutical ingredients and other ingredients used in the production of medicines.
Good Manufacturing Practice (GMP): Describes the minimum standards that a medicines manufacturer must meet in their production processes. For the example of GMP, here are the guidelines from the FDA and EU.
On the clinical side, the reduction of infections is crucial in high risk areas such as ultra clean operating suites, conventional operating suites and treatment rooms used for all types of surgical procedures but also in areas where obstetrics and maternity procedures are carried out. The second part is for the isolation, that is split into two categories:

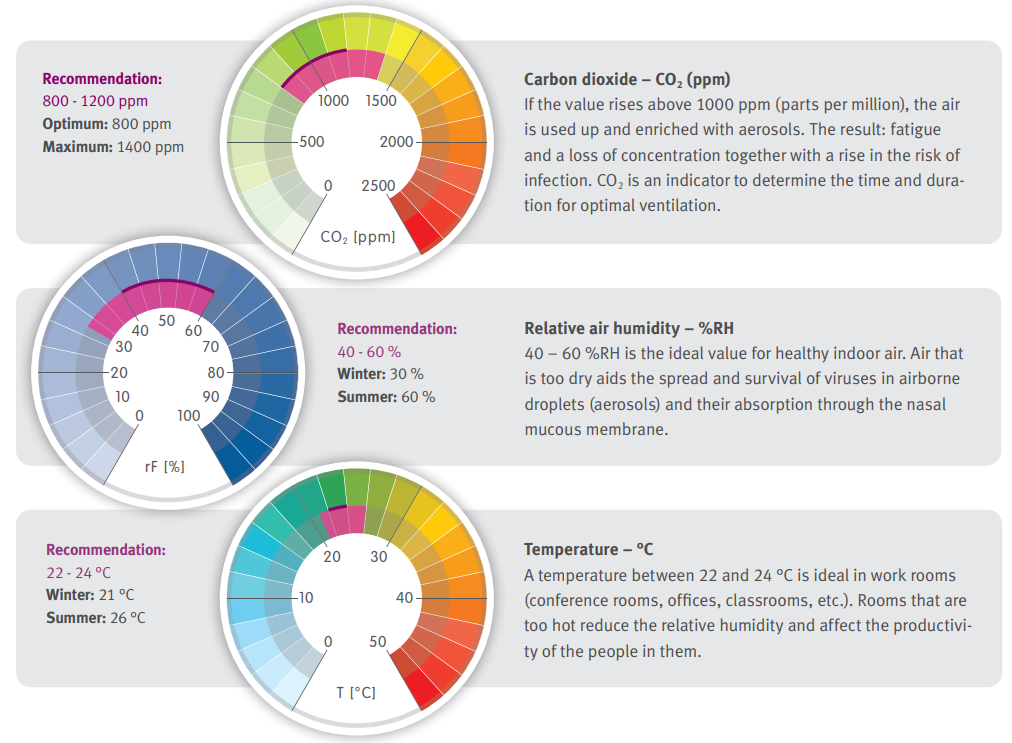
Personal comfort is an additional monitoring factor that can be taken into consideration when installing an environmental monitoring system. Along side temperature and relative humidity, parameters such as carbon dioxide (CO2) and volatile organic compounds (VOC) can also be monitored.
Rotronic recommends the following;
The Rotronic devices can be calibrated using the device’s display or within the corresponding software. Rotronic offer a range of calibration devices for temperature and relative humidity:
RMS features a calibration management tool, where all historical calibrations are documented and reminders for upcoming calibrations are communicated. Rotronic also offer calibration services:
The RMS software is validatable, so time to validate your continuous monitoring system. If you did the URS with the Rotronic documentation, then we can also offer the rest of the validation documentation, the validation master plan, the risk assessment, the functional requirement specifications, the configuration specification, the requirement traceability matrix, the validation script specification, the installation qualification, the operation qualification and the performance qualification of course followed by your tailor made training.
This validation model is based upon the GAMP5 standard and is recognised throughout the pharmaceutical world. The entire validation will prove that your system replies to your URS. Even if you didn't use the Rotronic URS, you can still use the Rotronic documentation as guidelines. With our team of trained professionals, Rotronic can carry out the validation of your system partially or fully if required
The Rotronic Monitoring System (RMS) is a GAMP©5 category 4 software combined with category 1 hardware, helping users monitor their GxP compliant applications, looking into the critical quality attributes and monitoring critical process parameters, helping focus on patient safety, product quality and data integrity and compliant to EudraLex Annex 11 and FDA 21 CFR Part 11.
Parameters
•Relative humidity: 0…100%rhRMS allows the user to configure each measuring points with a warning (pre-alarm) and an alarm. Both the warning and the alarm can be configured with a delay (for example for differential pressure) as well as a hysteresis. Notification repetitions can be setup to ensure that critical events are not forgotten, warnings and alarms can be sent via E-Mail, SMS and telephone call.
ISO 14644-2 offers quite an insight into alert and action levels as well as the generation of too many alerts. RMS will allow for specific users to adapt the various levels to ensure that optimal levels are maintained: all changes are documented within the audit trail, stating the user who made the changes, the time stamp and the values before and after the change.